In the last two articles about the Apple Watch's capability to detect atrial fibrillation, I made references to terminology ("false positive") that has its roots in Signal Detection Theory. Signal Detection Theory was developed as a means to determine the accuracy of early radar systems. The technique has migrated to communications systems, psychology, diagnostics and a variety of other domains where determining the presence or absence of something of interest is important especially when the signal to be detected would be presented within a noisy environment (this was particularly true of early radars) or when the signal is weak and difficult to detect.
Signal detection can be powerful tool to guide research methodologies and data analysis. I have used the signal detection paradigm in my own research both for the development of my research methodology and data analysis: planned and post-hoc analysis. In fact when I have taught courses in research methods and statistical analysis, I have used the signal detection paradigm as a way to convey detecting the effects of an experimental manipulation in your data.
Because I've mentioned issues related to signal detection and that it is a powerful tool for research and development, I decided to provide a short primer of signal detection.
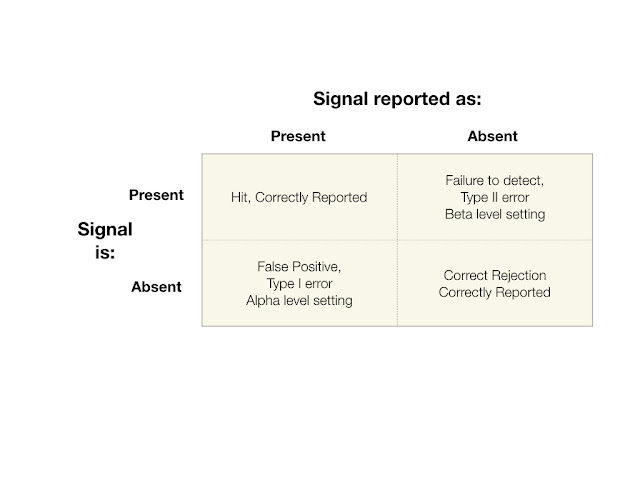
The central feature of signal detection is the two by two matrix shown below.
The signal detection process begins with a detection window or event. The window for detection could be a period of time or a specified occurrence such as a psychological test such as a rapid presentation of a stimulus and determine whether or not the subject of the experiment detected what was presented.
Or in the case of the Apple Watch, whether it detects atrial fibrillation. In devices such as the Apple Watch, how the system defines the detection window can be important. Since we have no information regarding how the Apple Watch atrial fibrillation detection system operates, it's difficult to determine how it determines its detection window.
During the window of detection, a signal may or may not be present. Each cell represents an outcome of a detection event. The possible outcomes are: 1: the signal was present and it was detected, a hit (upper left cell), 2: the signal was not present and the system or person correctly correctly reported no signal present (lower right cell), 3: the signal was absent, but erroneously reported as present, this is a Type I error (lower left cell) and 4: the signal was present, but reported as absent, this is a Type II error (upper right cell).
The object of any system is that the outcomes of detection events end up in outcome cells 1 and 2, that is, correctly reported. However, from a research standpoint, the error cells (Outcomes 3 and 4) are the most interesting and revealing.
Outcome 3: Type I Error
A Type I error is reporting that a signal is present when it was not. This is known as a "false alarm or false positive." The statistic for alpha which is the ratio of Outcome 3 over Total number of trials or detection events.
Outcome 4: Type II Error
A Type II error is reporting that a signal is not present when in fact it was present. This is a "failure to detect." The statistic for beta which is the ratio of Outcome 4 over Total number of trials or detection events.
If you're designing a detection system, the idea is to minimize both types of errors. However, no system is perfect and as such, it's important to determine what type of error is most acceptable, Type I or II because there are likely to be consequences either way.
Trade-off Between Type I and Type II Errors
In experimental research the emphasis has largely been on minimizing Type I errors, that is reporting an experimental effect when in actuality none was present. Increasing your alpha level, that is decreasing your acceptance of Type I errors, increases the likelihood of making a Type II error, reporting that an experimental effect was not present when in fact it was.
However, with medical devices, what type of error is of greater concern, Type I or Type II? That's a decision that will need to be made.
Before leaving this section, I should mention that the trade-off analysis between Type I and Type II errors is called Receiver-Operating-Characteristic Analysis or ROC-analysis. This is something that I'll discuss in a later article.
Signal detection can be powerful tool to guide research methodologies and data analysis. I have used the signal detection paradigm in my own research both for the development of my research methodology and data analysis: planned and post-hoc analysis. In fact when I have taught courses in research methods and statistical analysis, I have used the signal detection paradigm as a way to convey detecting the effects of an experimental manipulation in your data.
Because I've mentioned issues related to signal detection and that it is a powerful tool for research and development, I decided to provide a short primer of signal detection.
Signal Detection
The central feature of signal detection is the two by two matrix shown below.
The signal detection process begins with a detection window or event. The window for detection could be a period of time or a specified occurrence such as a psychological test such as a rapid presentation of a stimulus and determine whether or not the subject of the experiment detected what was presented.
Or in the case of the Apple Watch, whether it detects atrial fibrillation. In devices such as the Apple Watch, how the system defines the detection window can be important. Since we have no information regarding how the Apple Watch atrial fibrillation detection system operates, it's difficult to determine how it determines its detection window.
Multiple, Repeated Trials
Before discussing the meaning of the Signal Detection Matrix, it's important to understand that every matrix comes with multiple, repeated trials with a particular detection system, whether that detection system is a machine or a biological entity such as a person. Signal Detection Theory is grounded in probability theory, therefore, there is the requirement for multiple trials in order to create a viable and valid matrix.
The Four Cells of the Signal Detection MatrixDuring the window of detection, a signal may or may not be present. Each cell represents an outcome of a detection event. The possible outcomes are: 1: the signal was present and it was detected, a hit (upper left cell), 2: the signal was not present and the system or person correctly correctly reported no signal present (lower right cell), 3: the signal was absent, but erroneously reported as present, this is a Type I error (lower left cell) and 4: the signal was present, but reported as absent, this is a Type II error (upper right cell).
The object of any system is that the outcomes of detection events end up in outcome cells 1 and 2, that is, correctly reported. However, from a research standpoint, the error cells (Outcomes 3 and 4) are the most interesting and revealing.
Incorrect Report: Cells
Outcome 3: Type I Error
A Type I error is reporting that a signal is present when it was not. This is known as a "false alarm or false positive." The statistic for alpha which is the ratio of Outcome 3 over Total number of trials or detection events.
Outcome 4: Type II Error
A Type II error is reporting that a signal is not present when in fact it was present. This is a "failure to detect." The statistic for beta which is the ratio of Outcome 4 over Total number of trials or detection events.
If you're designing a detection system, the idea is to minimize both types of errors. However, no system is perfect and as such, it's important to determine what type of error is most acceptable, Type I or II because there are likely to be consequences either way.
Trade-off Between Type I and Type II Errors
In experimental research the emphasis has largely been on minimizing Type I errors, that is reporting an experimental effect when in actuality none was present. Increasing your alpha level, that is decreasing your acceptance of Type I errors, increases the likelihood of making a Type II error, reporting that an experimental effect was not present when in fact it was.
However, with medical devices, what type of error is of greater concern, Type I or Type II? That's a decision that will need to be made.
Before leaving this section, I should mention that the trade-off analysis between Type I and Type II errors is called Receiver-Operating-Characteristic Analysis or ROC-analysis. This is something that I'll discuss in a later article.
With Respect to the Apple Watch
Since I have no access into Apple's thinking when it was designing the Watch's atrial fibrillation software system, I can't know for certain the thinking that went into designing atrial fibrillation detection algorithm for the Apple Watch. However based on their own research, it seems that Apple made the decision to side on accepting false positives over false negatives -- although we can't be completely sure this is true because Apple did not do the research to determine rate that the Apple Watch failed to detect atrial fibrillation when it was know to be present.
With a "medical device" such as the Apple Watch, it would seem reasonable to side on accepting false positives over false positive. That is, to set your alpha level low. The hope would be that if the Apple Watch detected atrial fibrillation the owner of the watch would seek medical attention to determine whether or not a diagnosis of atrial fibrillation was warranted for receiving treatment for the condition. If the watch generated a false alarm, then there was no harm in seeking medical advice ... it would seem. The author of the NY Times article I cited in the previous article appears to hold to this point of view.
However ...
The problem with a system that generates a high rate of false alarms, is that all too often signals tend to be ignored. Consider the following scenario: an owner of an Apple Watch receives an indication that atrial fibrillation has been detected. The owner goes to a physician who reports that there's no indication of atrial fibrillation. Time passes and the watch reports again that atrial fibrillation has been detected. The owner goes back to the physician who give the owner the same report as before, no atrial fibrillation detected. What do you think will happen if the owner receives from the watch that atrial fibrillation has been detected? It's likely that the owner will just ignore the report. That would really be a problem for the owner if the owner had in fact developed atrial fibrillation. In this scenario the watch "cried wolf" too many times. And therein lies the problem with having a system that's adjusted to accepting a high rate of false alarms.
With a "medical device" such as the Apple Watch, it would seem reasonable to side on accepting false positives over false positive. That is, to set your alpha level low. The hope would be that if the Apple Watch detected atrial fibrillation the owner of the watch would seek medical attention to determine whether or not a diagnosis of atrial fibrillation was warranted for receiving treatment for the condition. If the watch generated a false alarm, then there was no harm in seeking medical advice ... it would seem. The author of the NY Times article I cited in the previous article appears to hold to this point of view.
However ...
The problem with a system that generates a high rate of false alarms, is that all too often signals tend to be ignored. Consider the following scenario: an owner of an Apple Watch receives an indication that atrial fibrillation has been detected. The owner goes to a physician who reports that there's no indication of atrial fibrillation. Time passes and the watch reports again that atrial fibrillation has been detected. The owner goes back to the physician who give the owner the same report as before, no atrial fibrillation detected. What do you think will happen if the owner receives from the watch that atrial fibrillation has been detected? It's likely that the owner will just ignore the report. That would really be a problem for the owner if the owner had in fact developed atrial fibrillation. In this scenario the watch "cried wolf" too many times. And therein lies the problem with having a system that's adjusted to accepting a high rate of false alarms.